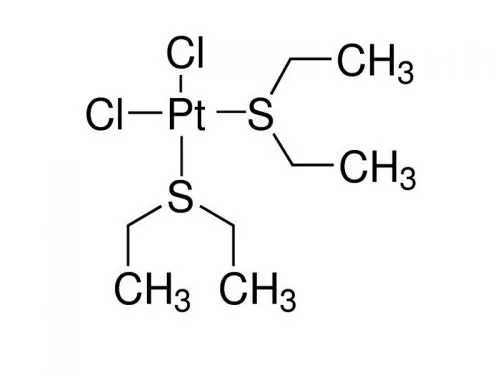
Platinum nanoparticles are formed by gas phase loading of the volatile platinum compounds on
metal-organic frameworks and by subsequent reduction with 100 bar hydrogen. For instance,
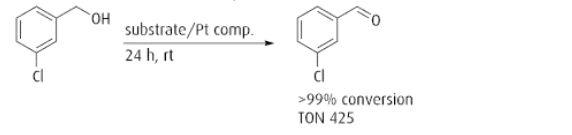
the Pt component shows high catalytic activity in solvent-free and base-free oxidation of
alcohols in the air at room temperature.
Reference: Chem. Eur. J. 2008, 14, 8204 (DOI: 10.1002/chem.200801043)
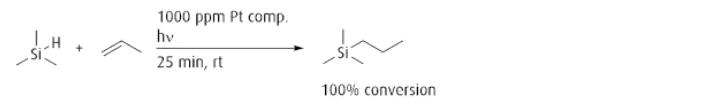
Trimethyl(methylcyclopentadienyl)platinum(IV) as an effective photoinitiator for hydrosilylation
reactions.
Reference: Inorg. Chem. 2004, 43, 6869 (DOI: 10.1021/ic0496222)
Trimethyl(methylcyclopentadienyl)platinum(IV) is often used as ALD and CVD precursor.
Reference: WO2010 033318 ; Electrochimica Acta 2012, 75, 101
(10.1016/j.electacta.2012.04.084)